FDA Approves Potential Lifesaver For Thousands Of Premature Babies
NEW YORK (CBSNewYork) - The Food and Drug Administration has just approved an amazing new device that could be a lifesaver for thousands of premature babies.
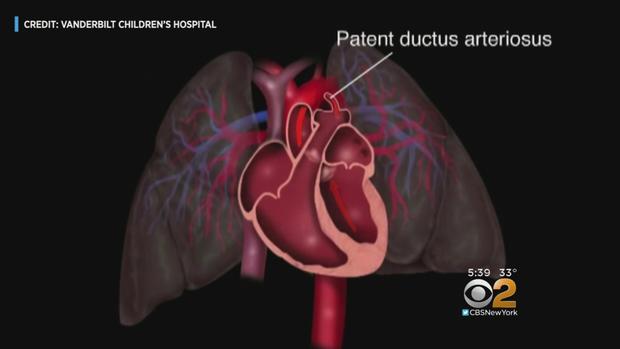
The little umbrella-like device closes an abnormal blood vessel.
You'd never know it by looking at the Felkner twins, but both were born three months prematurely. Judah did fine, but his sister Irie was gravely ill. She weighed less than two pounds, but her life-threatening problem was something called patent ductus ateriosus, or PDA.
A PDA is normal while the baby is in the womb. It shunts blood away from the lungs because the baby isn't breathing while in the womb. The PDA normally closes within a few days after birth, but in some babies it stays open, putting a huge strain on a tiny heart.
"We saw that her heart was enlarging in size. and more and more fluid was collecting in her lungs," said Dr. Aimee Armstrong of the Nationwide Children's Hospital.
Sometimes medications can induce the PDA to close, but sometimes it requires heart surgery, which is risky in a tiny preemie like Irie.
That's when the just FDA-approved tiny little metal device saved Irie's life. It's called the Amplatzer Piccolo, manufactured by Abbott. It is threaded up through a vein in the leg, through the heart and into the PDA, where it opens and stays, blocking the PDA and allowing blood to flow normally into the lungs.
"We were in and out in 30 minutes, you can't even see her incision, there's no scar," said Irie's mother Crissie Felkner.
Her doctor, who was an investigator in the clinical trials that to Amplatzer Piccolo approvals, says Irie's condition is completely resolved.
"Her heart disease is gone, her PDA is gone. She has no narrowings or issues in the heart. And she will not need to be followed long term because of the PDA," Dr. Armstrong said.
It doesn't take a doctor to see how well Irie is doing after her brush with a failing heart.
"Irie has no limitations, she doesn't have any. Outside of being small, she's a normal little baby, she's just now starting to walk and run around," her father Matt Felkner said.
A much larger version of the device has been in use for more than 20 years to close holes in the heart. Now this tiny version could help the nearly 12,000 preemies a year with significant PDA to avoid surgery.